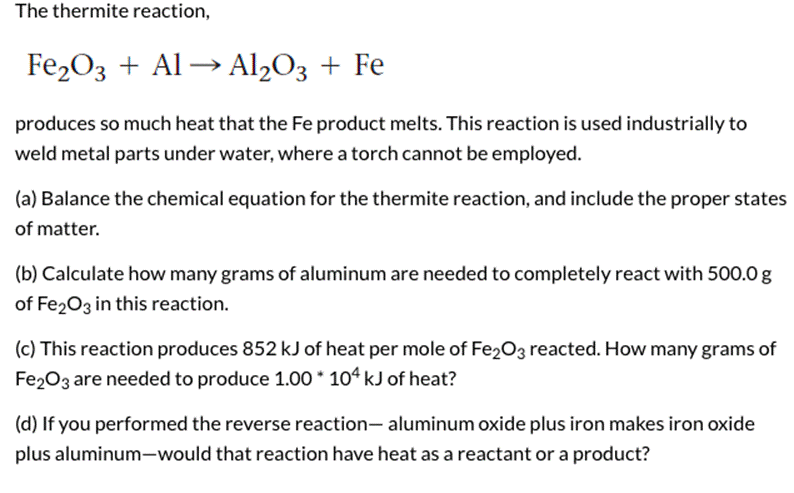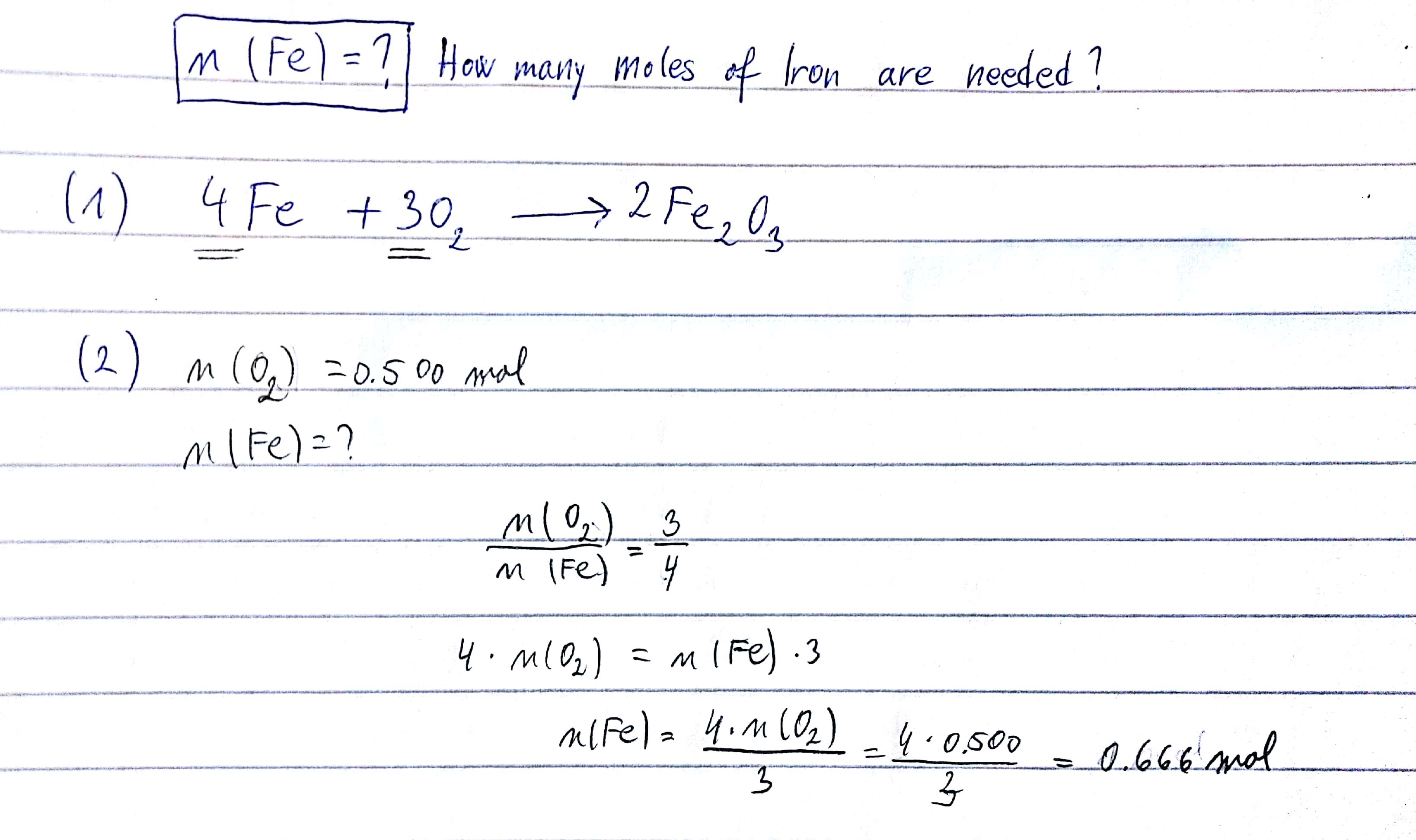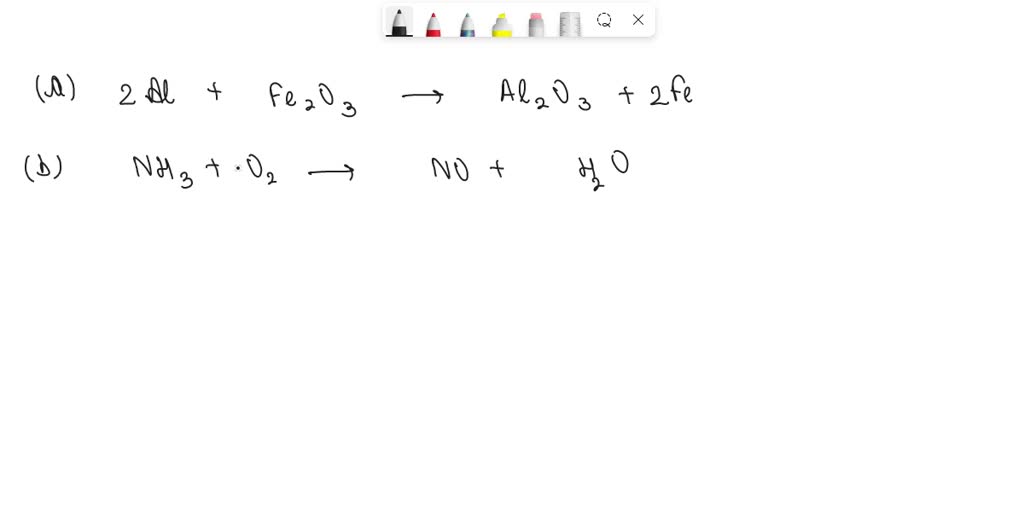
SOLVED: Balance this equations : (a) Al + Fe2O3 → Al2O3 + Fe (b) NH3 + O2 → NO + H2O (c) Ca + V2O5 → CaO + V

4) Name the substance oxidised , substance reduced ,oxidising and reducing agents Also balance the equation wherever necessary a) - Science - Chemical Reactions and Equations - 13670549 | Meritnation.com

Al + Fe2O3 → Al2O3 + Fe (Need to balance equation) How many grams of Fe can be produced when 10.0g of Al - brainly.com
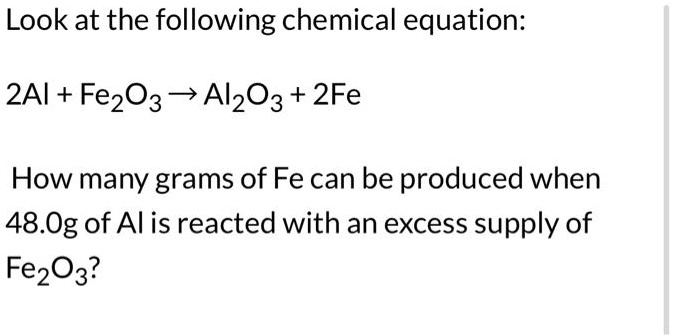
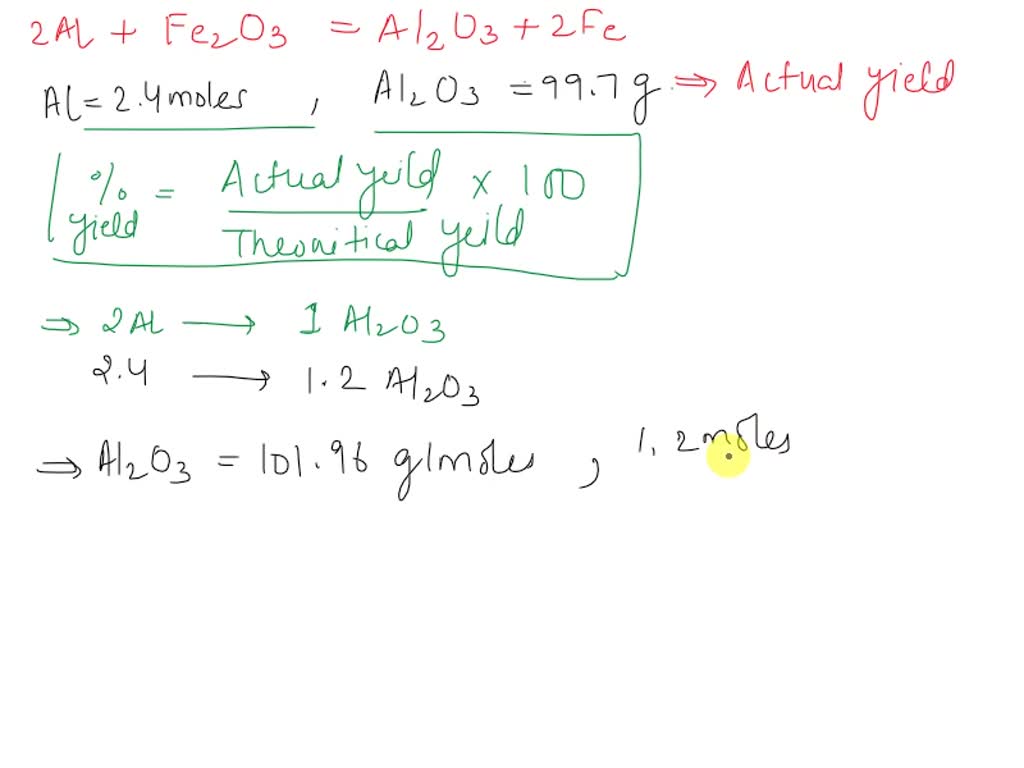
SOLVED: Look at the following chemical equation: 2Al + Fe2O3 -> Al2O3 + 2Fe How many grams of Fe can be produced when 48.0g of Al is reacted with an excess supply of Fe2O3?

Fe2O3+Al=Fe+Al2O3 balance the chemical equation by law of conservation of mass @my documentary. - YouTube

Um Tum Tune UI molecules relationship etc., Eg-1: Ale+Fe2O3 → A1,0368 + Fee (atomic masses of Al=270, Fe = 56U, and O=160) 2A1c +Fe2O3() ▻ A1,036 + 2Fe), is a balanced equation. (
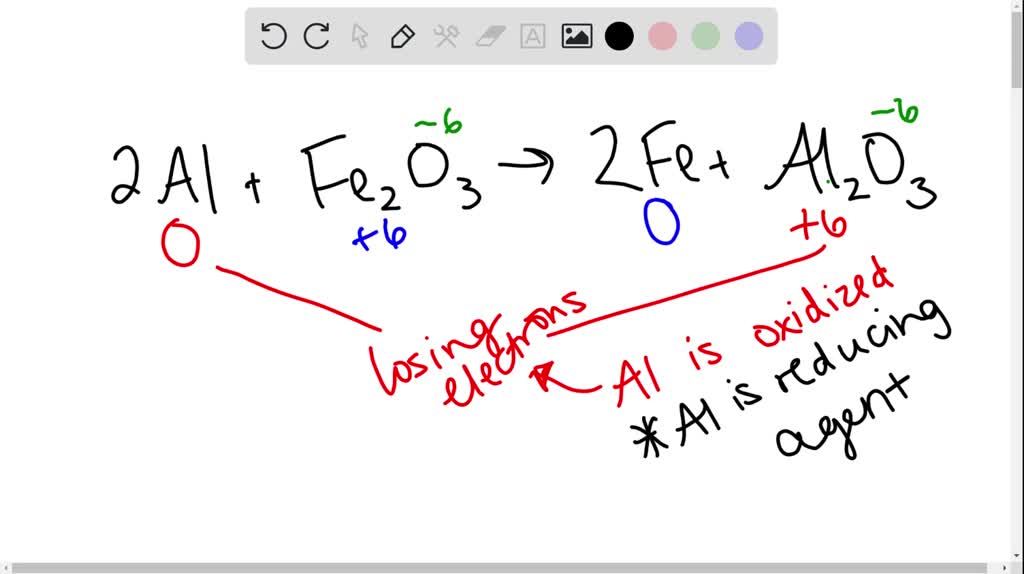
SOLVED: Consider the following oxidation-reduction reaction: Fe2O3(l) + 2Al(l) â†' Al2O3(l) + 2Fe(l) Which substance is the oxidizing agent and which one is the reducing agent? Select one: a. Oxidizing agent: Al2O3