
Balance the chemical equation: (1) Cu + HNO3 → Cu (NO3)2 + H2O + NO2 (2) NH3 + Cl2 → - Science - Chemical Reactions and Equations - 12597703 | Meritnation.com
a Cu + b HNO3 (dil.) —— gt; c Cu (No3)2 + d H2O + e No. How to solve this by algebraic method of balancing?

Algebraic method or a,b,c method for balance the equation. Cu+HNO3=Cu(NO3)2+NO2+H2O balance the equa - YouTube

SOLVED: You are given the reaction Cu + HNO3 → Cu(NO3)2 + NO + H2O. Half-reactions: First: 3 upper C u right arrow 3 upper C u superscript 2 plus, plus 6
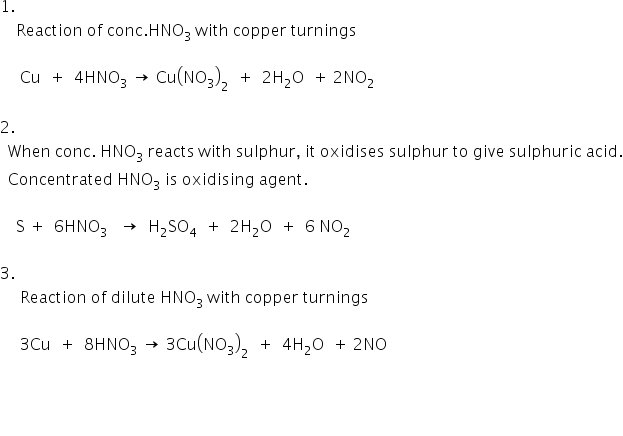
1) write a balanced equation for the reaction of conc.HNO3 when added to copper turnings kept in a beaker.2) write a balanced equation for the reaction of sulphur and hot concentrated nitric
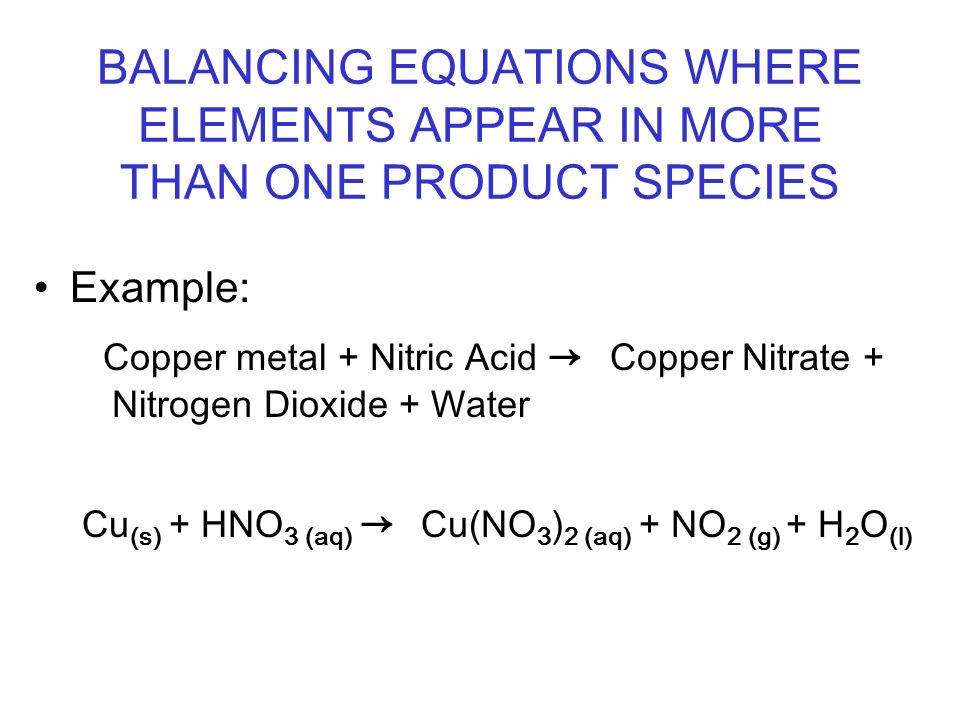





![Solved Balance the following equation [?] Cu+ [?] HNO3→ [?] | Chegg.com Solved Balance the following equation [?] Cu+ [?] HNO3→ [?] | Chegg.com](https://media.cheggcdn.com/study/5a9/5a986382-b1f5-4058-9dc1-bae2572aa503/image)



2%20+%20NO%20+%20H2O%20reaction.jpg?ezimgfmt=rs:323x202/rscb1/ngcb1/notWebP)

