
SOLVED: If 56.3 g of benzene (C6H6) are combusted with excess oxygen, what mass of carbon dioxide will be obtained? C6H6+O2⋯CO2+H2O

C6H6 +O2 ------}CO2 + H2O balance By oxi Methods - Chemistry - Redox Reactions - 13233357 | Meritnation.com
![Which set of conditions are correct for the reaction below? 2C_3H_8+7O_2to 6CO+8H_2O A. co [algebra] Which set of conditions are correct for the reaction below? 2C_3H_8+7O_2to 6CO+8H_2O A. co [algebra]](https://p16-ehi-va.gauthmath.com/tos-maliva-i-ejcjvp0zxf-us/416b1902cada4902a7141d7b6e990ae8~tplv-ejcjvp0zxf-webp.webp)
Which set of conditions are correct for the reaction below? 2C_3H_8+7O_2to 6CO+8H_2O A. co [algebra]

SOLVED: Balance the equation by inserting coefficients as needed: equation: C6H6 + O2 -> CO2 + H2O Classify the reaction: combustion
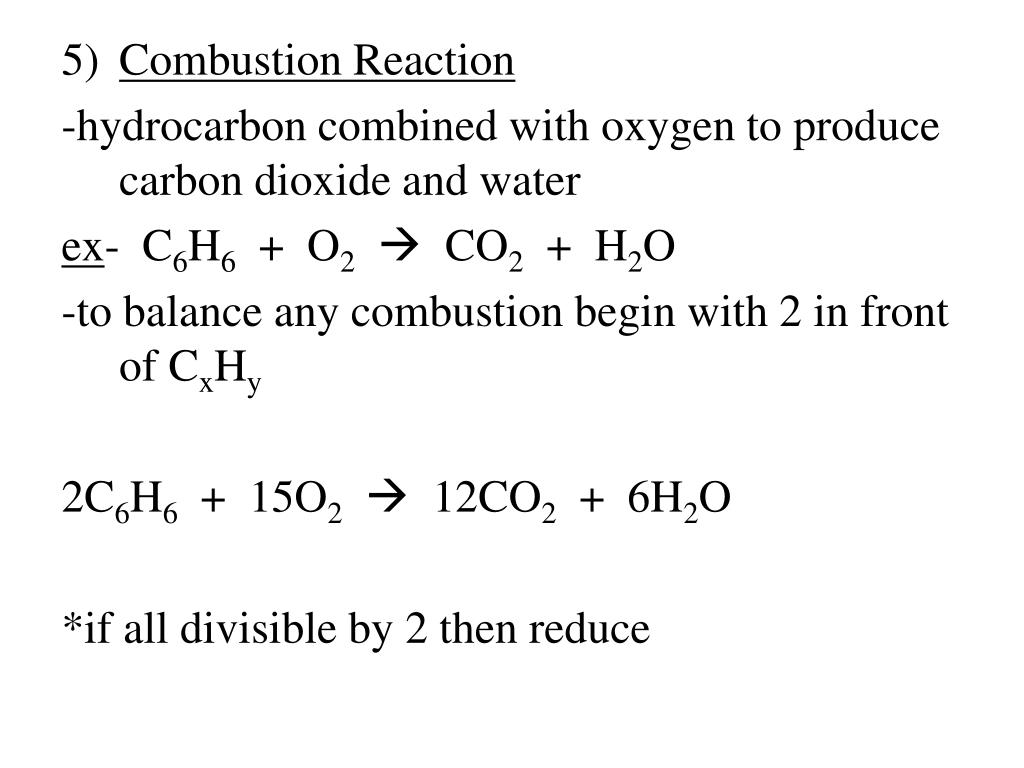
PPT - Chemical Equations reactants products reactant - starting substance in a chemical reaction PowerPoint Presentation - ID:5812787

C6H6+O2=CO2+H2O balance the chemical equation. c6h6+o2=co2+h2o benzene and oxygen reaction - YouTube
how to balance chemical equation c6h6+o2 arrow mark co2+h20 but. i tried and in RHS why should we put 12 next to carbon instead of that we can put put 3 so







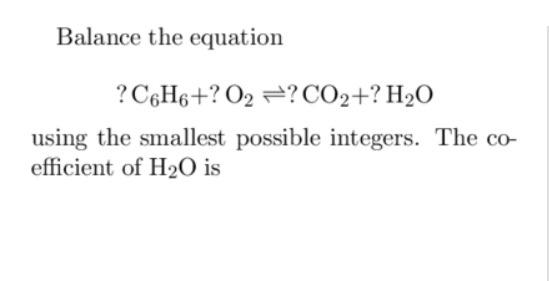


![Tamil] Balance the following equation by oxidation number method Tamil] Balance the following equation by oxidation number method](https://d10lpgp6xz60nq.cloudfront.net/physics_images/FM_CHE_XI_V01_C01_E04_188_S01.png)
